|
There are numerous studies measuring the benefits of
magnets to improve the quality of life. Speaking in general terms,
most studies showed 65 to 75 percent of the people studied recognized
benefits from magnets. There were a smaller number of people on
placebos that also recognized benefits. And in general about 25
percent of the people could not recognize any benefits. However
there are cases where people could not recognize the benefits until they
were off the study for a week and immediately purchased magnetic jewelry
to regain the benefits of magnetic therapy. The benefits are subtle
and every one has a different pain level. We suspect in many cases
people don't recognize the benefits because they are expecting more
immediate and dramatic results. However we recognize that some
people do not benefit from magnetic therapy. Following are some
summaries of the many studies observed.
[ Bracelets-Women's ]
[ Bracelets - Men's ] [
Necklaces ] [
Pet Collars ] [ Wraps ]
[ Clasps ]
[
Stainless & Titanium ] [
Super High Strength ] [ Anklets ]
[ Hip Pain ]

FDA NEWS RELEASE
For Immediate Release: Dec. 13, 2013
Media Inquiries: Jennifer Rodriguez, 301-796-8232, jennifer.rodriguez@fda.hhs.gov
Consumer Inquiries: 888-INFO-FDA
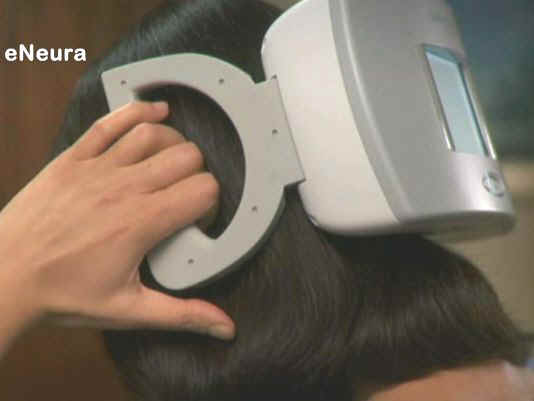
FDA allows marketing of first device to relieve migraine
headache pain
The U.S. Food and Drug Administration today allowed marketing of the
Cerena Transcranial Magnetic Stimulator (TMS), the first device to
relieve pain caused by migraine headaches that are preceded by an
aura: a visual, sensory or motor disturbance immediately preceding the
onset of a migraine attack.
Migraine headaches are characterized by intense pulsing or throbbing
pain in one area of the head accompanied by nausea and/or vomiting and
sensitivity to light and sound. A migraine can last anywhere between
four and 72 hours when untreated. These debilitating headaches affect
approximately 10 percent of people worldwide and are three times more
common in women than in men. About one third of people with migraines
experience an aura.
“Millions of people suffer from migraines and this new device
represents a new treatment option for some patients,” said Christy
Foreman, director of the Office of Device Evaluation in the FDA’s
Center for Devices and Radiological Health.
The Cerena TMS is a prescription device used after the onset of pain
associated with migraine headaches preceded by an aura. Using both
hands to hold the device against the back of the head, the user
presses a button to release a pulse of magnetic energy to stimulate
the occipital cortex in the brain, which may stop or lessen the pain
associated with migraine headaches preceded by an aura.
The FDA reviewed the data for the Cerena TMS through the de novo
premarket review pathway, a regulatory pathway for some low- to
moderate-risk medical devices that are not substantially equivalent to
an already legally marketed device.
The FDA reviewed a randomized control clinical trial of 201 patients
who had mostly moderate to strong migraine headaches and who had auras
preceding at least 30 percent of their migraines. Of the study
subjects, 113 recorded treating a migraine at least once when pain was
present. Analysis of these 113 subjects was used to support marketing
authorization of the Cerena TMS for the acute treatment of pain
associated with migraine headache with aura.
The study showed that nearly 38 percent of subjects who used the
Cerena TMS when they had migraine pain were pain-free two hours after
using the device compared to about 17 percent of patients in the
control group. After 24 hours, nearly 34 percent of the Cerena TMS
users were pain-free compared to 10 percent in the control group.
The study did not show that the Cerena TMS is effective in relieving
the associated symptoms of migraine, such as sensitivity to light,
sensitivity to sound, and nausea. The device is for use in people 18
years of age and older. The study did not evaluate the device’s
performance when treating types of headaches other than migraine
headaches preceded by an aura.
Adverse events reported during the study were rare for both the device
and the control groups but included single reports of sinusitis,
aphasia (inability to speak or understand language) and vertigo
(sensation of spinning). Dizziness may be associated with the use of
the device.
Patients must not use the Cerena TMS device if they have metals in the
head, neck, or upper body that are attracted by a magnet, or if they
have an active implanted medical device such as a pacemaker or deep
brain stimulator. The Cerena TMS device should not be used in patients
with suspected or diagnosed epilepsy or a personal or family history
of seizures. The recommended daily usage of the device is not to
exceed one treatment in 24 hours.
FDA Approves Magnetic Treatment for Depression
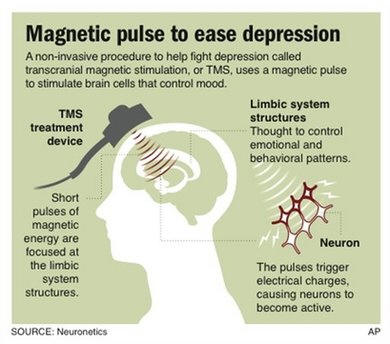

Mon Oct 20, 2012, 5:38 PM ET
Graphic shows how magnetic
stimulation is used to treat depression;
WASHINGTON – The government has approved the first
noninvasive brain stimulator to treat depression — a device that beams
magnetic pulses through the skull.
If it sounds like science-fiction, well, those
woodpecker-like pulses trigger small electrical charges that spark
brain cells to fire. Yet it doesn't cause the risks of surgically
implanted electrodes or the treatment of last resort,
shock therapy.
Called transcranial magnetic stimulation or TMS, this
gentler approach isn't for everyone. The
Food and Drug
Administration approved Neuronetics Inc.'s NeuroStar therapy
specifically for patients who had no relief from their first
antidepressant, offering them a different option than trying pill after
pill.
"We're opening up a whole new area of medicine," says
Dr. Mark George of the
Medical University of South Carolina in Charleston, who helped
pioneer use of TMS in depression.
"There's a whole field now that's
moving forward of noninvasive electrical stimulation of the brain."
While there's a big need for innovative approaches — at
least one in five depression patients is treatment-resistant — the
question is just how much benefit TMS offers.
The FDA
cleared the prescription-only NeuroStar based on data that found
patients did modestly better when treated with TMS than when they
unknowingly received a sham treatment that mimicked the magnet. It was a
study fraught with statistical questions that concerned the agency's own
scientific advisers.
For a more clear answer, the
National Institutes of
Health has an independent study under way now that tracks 260
patients and may have initial results as early as next year.
Quantifying the benefit is key, considering the price
tag. TMS is expected to cost $6,000 to $10,000, depending on how many
treatments a patient needs, says Dr. Philip Janicak of
Rush University Medical
Center in Chicago, who helped lead the NeuroStar study. That's
far more expensive than medication yet thousands of dollars cheaper than
invasive depression devices.
Neuroscientists have been using TMS for years as a
research tool in brain studies. Zap a powerful magnet over a certain
spot on the head — where motion is controlled — and someone's arm can
suddenly, involuntarily, lash out. Beyond the "wow" factor, magnetized
pulses were triggering brain activity.
The question was how to harness that activity in a way
that might improve disease. TMS also is being studied in stroke
rehabilitation and other brain disorders.
"Nobody thought this would work; it was a crazy idea. I
had to do it at 6 in the morning before the real scientists came in,"
South Carolina's George laughs as he recalls work he began in 1993.
But, "the brain is an electrical organ," George adds,
explaining the rationale. "Electricity is the currency of the brain.
It's how the brain does what it does."
For depression, psychiatrists aim the magnet at the left
front of the head, the
prefrontal cortex. Since everyone's brain is different, they
first zap the top of the head to find a patient's motor-control region,
and then carefully move 5 centimeters forward. Then, the NeuroStar beams
about 3,000 pulses a minute during a 40-minute treatment, done about
five times a week for up to six weeks.
The theory: Stimulating
brain cells in the
prefrontal cortex triggers a chain reaction that also stimulates deeper
brain regions involved with mood.
TMS did prove to be very safe: Patients in the NeuroStar
study suffered no seizures or memory problems like
shock therapy can cause, or other reactions throughout the body.
The chief complaint
from the sessions was headaches.
The
FDA cleared the device after focusing just on a subset of the
patients initially enrolled — 164 who had failed one antidepressant
during their current bout of depression, not those who were more
severely treatment-resistant.
What's a modest benefit? About 24 percent who got TMS
scored significantly better on standard depression measures after six
weeks, compared with 12 percent who got the sham, says Janicak. That's
about as well as patients respond to a single antidepressant, he says.
Some reported remarkable improvement.
"One day it was like a light switch went off," says
Steve Newman, 60,
of Washington, D.C., who enrolled in the NeuroStar study at the
University of
Pennsylvania in 2005.
Newman had suffered repeated bouts of depression since
he was a teenager, and drug after drug barely blunted it. He was
considering shock therapy when he heard about TMS.
After two weeks of treatment, Newman was wondering if he
was getting the sham — when suddenly, he started feeling lots better,
and doctors spotted a corresponding major improvement in his depression
measurements.
"I was awake. I was there," says Newman who said he
still gets what he calls a "maintenance dose" of TMS about once a month.
___
EDITOR's NOTE — Lauran Neergaard covers health and
medical issues for The Associated Press in Washington.
***************************************************************************************
MAGNETIC DISCS COULD KILL CANCER CELLS
PARIS (AFP) – Tiny magnetic discs just a millionth of a
metre in diameter could be used to used to kill
cancer cells, according to a study published on Sunday.
Laboratory tests found the so-called "nanodiscs", around
60 billionths of a metre thick, could be used to disrupt the membranes
of cancer cells, causing them to self-destruct.
The discs are made from an iron-nickel alloy, which move
when subjected to a magnetic field, damaging the cancer cells, the
report published in Nature Materials said.
One of the study's authors, Elena Rozhlova of
Argonne National Laboratory in the United States, said subjecting
the discs to a low magnetic field for around ten minutes was enough to
destroy 90 percent of cancer cells in tests.
In a commentary on the report, Jon Dobson of
Keele University in Britain said antibodies could be used to
direct the discs towards tumour cells.
"This provides an elegant and rapid technique for
targeting tumour destruction without the side effects associated with
systemic treatments such as chemotherapy," Dobson wrote.
**************************************************************************************************************************
CHRONIC PAIN
Efficacy of static
magnetic field therapy in chronic pelvic pain: a double-blind pilot
study.
Brown CS,
Ling FW, Wan JY, Pilla AA.
Department
of Pharmacy Practice and Pharmacoeconomics, University of Tennessee
Health Sciences Center, Memphis, USA. csbrown@utmem.edu
OBJECTIVE:
The aim of the study was to
determine the efficacy of static magnetic field therapy for the
treatment of chronic pelvic pain (CPP) by measuring changes in pain
relief and disability.
STUDY
DESIGN: Thirty-two patients with CPP completed 2 weeks and 19 patients
completed 4 weeks of randomized double-blind placebo-controlled
treatment at a gynecology clinic. Active (500 G) or placebo magnets were
applied to abdominal trigger points for 24 hour per day. The McGill Pain
Questionnaire, Pain Disability Index, and Clinical Global Impressions
Scale were outcome measures.
RESULTS:
Patients receiving active magnets
who completed 4 weeks of double-blind treatment had significantly lower
Pain Disability Index (P <.05), Clinical Global
Impressions-Severity (P <.05), and Clinical Global
Impressions-Improvement (P <.01) scores than those receiving placebo
magnets, but were more likely to correctly identify their treatment (P
<.05).
CONCLUSION:
SMF therapy significantly
improves disability and may reduce pain when active magnets are worn
continuously for 4 weeks in patients with CPP, but blinding
efficacy is compromised.
Am J Obstet
Gynecol 2002 Dec;187(6):1581-7
**************************************************************************************************************************
ARTHRITIS
The use
of magnetotherapy in diseases of the musculoskeletal system.
Sadlonova J, Korpas J.
Ist Dpt of
Internal Medicine, Jessenius Faculty of Medicine, Comenius University,
Martin, Slovakia. bll@fmed.uniba.sk
Therapeutic
application of pulsatile electromagnetic field in disorders of motility
is recently becoming more frequent. Despite this fact information about
the effectiveness of this therapy in the literature are rare. The aim of
this study was therefore the treatment of 576 patients who suffered from
vertebral syndrome, gonarthritis and coxarthritis. For application of
pulsatile electromagnetic field MTU 500H Therapy System was used.
Pulsatile electromagnetic field had a frequency value of 4.5 mT in all
studied groups and magnetic induction value 12.5-18.75 mT in the 1st
group. In the 2nd group the intensity was 5.8-7.3 mT and in the 3rd
group it was 7.6-11.4 mT. The time of inclination/declination in the 1st
group was 20/60 ms, in the 2nd group 40/80 ms and in the 3rd group 40/90
ms. The electromagnetic field was applied during 10 days. In the 1st-3rd
day during 20 minutes and in the 4th-10th day during 30 minutes. The
therapy was repeated in every patient after 3 months with values of
intensity higher by 50%. In the time of pulsatile electro-magnetotherapy
the patients were without pharmacotherapy or other physiotherapy.
The application of pulsatile
electromagnetic field is a very effective therapy of vertebral syndrome,
gonarthritis and coxarthritis.
The results have shown that the therapy was more effective in patients
suffering from gonarthrosis, than in patients with vertebral syndrome
and least effective in patients with coxarthosis. Owing to regression of
oedema and pain relief the motility of patients improved. (Tab. 3, Ref.
19.)
Bratisl Lek Listy. 1999 Dec;100(12):678-81.
***********************************************************************************************************************
BACTERIA
Effect
of static magnetic fields on bacteria: Streptococcus mutans,
Staphylococcus aureus, and Escherichia coli.
Kohno M,
Yamazaki M, Kimura I I, Wada M.
Application
and Research Center, Analytical Instruments Division, JEOL LTD., 1-2
Musashino 3-Chome, Akishima, 196-8558, Tokyo, Japan
Biological
effect of static magnetic field was investigated by using ferrite
magnets to conduct a magnetic field exposure experiment on three species
of bacteria: Streptococcus mutans, Staphylococcus aureus, and
Escherichia coli. The effects were evaluated by culturing the bacteria
and determining their growth rate, the maximum numbers of bacteria, and
[3H]-thymidine incorporation.
The results showed that the ferrite magnet caused strength-dependent
decreases in the growth rate and growth maximum number of bacteria for
S. mutans and S. aureus when cultured under anaerobic conditions,
but that their growth was not inhibited under aerobic conditions. In
addition, [3H]-thymidine was added after culturing each of the species
of bacteria for 18 h. After that, culture was continued until 24 h, and
changes in [3H]-thymidine incorporation were investigated. But no effect
of the magnetic fields was detected. These findings suggested that
oxygen related to growth the cases of S. mutans, S. aureus. However, no
growth effects were detected on E. coli cultures.
Pathophysiology. 2000 Jul;7(2):143-148.
************************************************************************************************************************
CANCER AND CHEMOTHERAPY
Effect of magnetic
fields on human and rodent cancer cell survival.
Tata, D.,
Vanhoutten, N., Brook, C., &TrItton T.
In a
laboratory study, several rodent and human cancer cell types were
exposed to permanent magnetic fields for one hour to determine what
percent of the cells would survive compared to unexposed cells. The
permanent magnetic field was extremely strong (11.6 Tesla = 116,000
gauss) and was generated by sophisticated equipment. Some of the
surviving cell fractions included 25% for human breast carcinoma, 40%
for human ovarian carcinoma, and 4% for human mouth carcinoma.
Non-Invasive permanent magnetic
field modality induces lethal effects on several rodent and human
cancers.
In Vitro.
Proceedings of the American Association for Cancer Research, 1994; 35,
386.
*************************************************************************************************************************
CARDIOVASCULAR
Static magnetic
field influence on rat brain function detected by heart rate monitoring.
Veliks V,
Ceihnere E, Svikis I, Aivars J.
Faculty of
Biology, University of Latvia, Riga, Latvia.
The aim of
the present study was to identify the effects of a static magnetic field
(SMF) on rat brain structures that control autonomic functions,
specifically heart rate and heart rhythmicity. The experiments were
carried out on 44 male Wistar rats under ketamine-xylazine anesthesia.
SMF was induced using samarium-cobalt fused magnets (20 x 20 x 10 mm in
size) placed bitemporally. Magnetic induction intensity was 100 mT on
the surface of the head. Duration of magnetic field application was 15
min. An electrocardiogram was recorded from limb lead II, and both heart
rate (average duration of cardiac cycles) and heart rhythmicity were
analyzed before and after SMF application.
SMF evoked changes in both heart
rate and rhythm in 80% of the animals; the predominant effects were
bradycardia and disappearance of respiratory sinus arrhythmia.
However, the effectiveness of SMF in large measure depends on both
functional peculiarities and functional activities of brain autonomic
centers. Bioelectromagnetics 25:211-215, 2004. Copyright 2004 Wiley-Liss,
Inc.
Bioelectromagnetics. 2004 Apr;25(3):211-5.
*************************************************************************************************************************
FIBROMYALGIA
Biochemical study
of human periodontal ligament: preparation of cell attachment materials
induced by pulsed electromagnetic fields.
Kim KT.
Department
of Oral Biochemistry, Kanagawa Dental College, Japan.
The
periodontium, especially the periodontal ligament and alveolar bone, are
tissues constantly subjected to physical stress such as occlusion and
mastication. This study was designed to explore the effect of the pulsed
electromagnetic fields (PEMF) on the cell attachment and the spread of
human periodontal ligament fibroblasts (HPLF) and rat osteoblasts (ROB).
PEMF are categorized as one type of mechanical stress. HPLF were
obtained by the explantation method described by Saito et al. They were
then subcultured in Dulbecco's modified Eagle's medium (D-MEM) and
supplemented with 2 mg/ml dialyzed fetal calf serum protein (FCSP), 50
micrograms/ml ascorbic acid and penicillin/streptomycin after
trypsinization. ROB were isolated from a two-day-old rat calvaria by the
sequential bacterial collagenase digestion method described by Dziak and
Brand and were subcultured in D-MEM supplemented with FCSP, ascorbic
acid and penicillin/streptomycin. After the confluent HPLF were cultured
with serum-free MCDB 107 medium, the quiescent HPLF were exposed with or
without PEMF for 24 hr. This was followed by the collection of the
control conditioned medium (C-CM) and PEMF exposed conditioned medium (PEMF-CM).
The cell attachment assay was performed so that the hydrophobic 24
multiwells were coated with the whole conditioned medium or fractionated
conditioned medium by a PO-60K column. After coating, heat inactivated
BSA blocked nonspecific sites for cell adhesion, and 3H-TdR labeled HPLF
or ROB were cultured on the precoated wells. The activity of cell
attachment and spreading was determined by the radioactivity of 3H-TdR
using a scintillation counter. The characters of cell attachment factors
derived from HPLF were hydrophobic, heat labile and proteolytic enzyme
digestible. In addition, the fractionated PEMF-CM enhanced the spreading
activity of ROB. PEMF induced the 10 KDa which can enhance the HPLF and
ROB spreading. Therefore, the
cell attachment and spreading factors secreted by human periodontal
ligament fibroblasts exposed with pulsed electromagnetic fields may
regulate human periodontal ligament fibroblasts and also rat osteoblasts.
Bull
Kanagawa Dent Coll. 1990 Sep;18(2):89-98
***************************************************************************************************************************
HEALING
Clinical
effectiveness of magnetic field therapy--a review of the literature.
Quittan M,
Schuhfried O, Wiesinger GF, Fialka-Moser V.
Universitatsklinik fur Physikalische Medizin und Rehabilitation, Wien.
michael.quittan@akh-wien.ac.at
To verify
the efficacy of electromagnetic fields on various diseases we conducted
a computer-assisted search of the pertinent literature. The search was
performed with the aid of the Medline and Embase database (1966-1998)
and reference lists. Clinical trials with at least one control group
were selected. The selection criteria were met by 31 clinical studies.
20 trials were designed double-blind, randomised and placebo-controlled.
The studies were categorised by indications. Electromagnetic fields were
applied to promote bone-healing, to treat osteoarthritis and
inflammatory diseases of the musculoskeletal system, to alleviate pain,
to enhance healing of ulcers and to reduce spasticity.
The action on bone healing and
pain alleviation of electromagnetic fields was confirmed in most of the
trials. In the treatment of other disorders the results are
contradictory. Application times varied between 15 minutes and 24 hours
per day for three weeks up to eighteen months. There
seems to be a relationship between longer daily application time and
positive effects particular in bone-healing. Patients were
treated with electromagnetic fields of 2 to 100 G (0.2 mT to 10 mT) with
a frequency between 12 and 100 Hz. Optimal dosimetry for therapy with
electromagnetic fields is yet not established.
Acta Med
Austriaca. 2000;27(3):61-8.
**************************************************************************************************************************
INCONTINENCE
Extracorporeal
magnetic stimulation for the treatment of stress and urge incontinence
in women--results of 1-year follow-up.
Unsal A,
Saglam R, Cimentepe E.
Department
of Urology, School of Medicine, Fatih University, Ciftlik Cd. No: 57,
TR-06510 Emek, Ankara, Turkey. unsalali@hotmail.com
OBJECTIVE:
To evaluate the clinical efficacy of extracorporeal magnetic stimulation
for the treatment of stress and urge urinary incontinence in women.
MATERIAL
AND METHODS: A total of 35 patients with stress incontinence and 17 with
urge incontinence were enrolled in this study. All patients were
evaluated by means of a detailed history of incontinence, a gynecologic
examination, urine culture, urinary system ultrasound and a urodynamic
study. All patients were asked to keep a 3-day voiding diary. A
pad-weighing test was done for each patient at their first visit. For
treatment, the patients were seated on a special chair containing a
magnetic field generator. Pelvic floor muscle stimulation was performed
for 20 min (10 min at 5 Hz and 10 min at 50 Hz) twice a week for a total
of 8 weeks. The mean follow-up period was 16.8 months (range 12-32
months). A total of 44 patients completed 1 year of follow-up and were
re-evaluated by means of voiding diary, pad-weighing test and
cystometric study.
RESULTS: Of
the 44 patients, 11 (38%) with stress incontinence and 6 (40%) with urge
incontinence were cured 1 year after the treatment. In addition, there
was an improvement in symptoms in 12 patients (41%) in the stress group
and 7 (47%) in the urge group. Pad weight was reduced from 15.4 to 5.8 g
in the stress group and from 12.4 to 4.7 g in the urge group (p = 0.000
and 0.001, respectively). Mean Valsalva leak point pressure was
increased from 87.3 +/- 15.9 to 118.0 +/- 11.0 cmH (2) O in the stress
group (p = 0.000).
CONCLUSIONS: Extracorporeal
magnetic stimulation therapy offers a non-invasive, effective and
painless treatment for stress and urge incontinence in women.
Scand J
Urol Nephrol. 2003;37(5):424-8
**************************************************************************************************************************
MIGRAINE & HEADACHE
Treatment of
migraine with pulsing electromagnetic fields: a double-blind,
placebo-controlled study.
Sherman RA,
Acosta NM, Robson L.
Orthopedic
Surgery Service, Madigan Army Medical Center, Tacoma, WA 98431, USA.
The effect
of exposure to pulsing electromagnetic fields on migraine activity was
evaluated by having 42 subjects (34 women and 8 men), who met the
International Headache Society's criteria for migraine, participate in a
double-blind, placebo-controlled study. Each subject kept a 1-month,
pretreatment, baseline log of headache activity prior to being
randomized to having either actual or placebo pulsing electromagnetic
fields applied to their inner thighs for 1 hour per day, 5 days per
week, for 2 weeks. After exposure, all subjects kept the log for at
least 1 follow-up month. During the first month of follow-up, 73% of
those receiving actual exposure reported decreased headaches (45% good
decrease, 14% excellent decrease) compared to half of those receiving
the placebo (15% worse, 20% good, 0% excellent). Ten of the 22 subjects
who had actual exposure received 2 additional weeks of actual exposure
after their initial 1-month follow-up. All showed decreased headache
activity (50% good, 38% excellent). Thirteen subjects from the actual
exposure group elected not to receive additional exposure. Twelve of
them showed decreased headache activity by the second month (29% good,
43% excellent). Eight of the subjects in the placebo group elected to
receive 2 weeks of actual exposure after the initial 1-month follow-up
with 75% showing decreased headache activity (38% good, 38% excellent).
In conclusion, exposure of the inner thighs to pulsing electromagnetic
fields for at least 3 weeks is an effective, short-term intervention for
migraine, but not tension headaches.
Headache.
1999 Sep;39(8):567-75
**************************************************************************************************************************
MULTIPLE SCLEROSIS
Weak
electromagnetic fields attenuate tremor in multiple sclerosis.
Sandyk R,
Dann LC.
NeuroCommunication Research Laboratories, Danbury, CT 06811, USA.
It has been
estimated that about 75% of patients diagnosed with multiple sclerosis
(MS) have tremor which can be exceedingly disabling. The most common
tremor observed in patients with MS is a cerebellar intention tremor
('kinetic tremor') although postural tremor ('static tremor') is also
common and often extremely incapacitating. Currently there is no
effective medical treatment for the tremor of MS which, in some severe
cases, may be abolished by stereotactic thalamotomy. It was reported
recently that extracranial application of brief AC pulsed
electromagnetic fields (EMFs) in the picotesla (pT) range produced
improvement in motor and cognitive functions in patients with MS. The
present communication concerns three MS patients with a chronic
progressive course of the disease (mean age: 39.3 +/- 8.3 years; mean
duration of illness: 11.3 +/- 3.2 years) in whom brief external
applications of pulsed EMFs of 7.5 pT intensity reduced intention and
postural tremors resulting in significant functional improvement.
The report suggests that these
extremely low intensity electromagnetic fields are beneficial also in
the treatment of tremors in MS and that this treatment may serve as an
alternative method to stereotactic thalamotomy in the management of
tremor in MS. The mechanisms by which EMFs attenuate the tremors
of MS are complex and are thought to involve augmentation of GABA and
serotonin (5-HT) neurotransmission in the cerebellum and its outflow
tracts.
Int J
Neurosci. 1994 Dec;79(3-4):199-212.
**************************************************************************************************************************
OSTEOPOROSIS
The effect of
pulsed electromagnetic fields on osteoporosis at the knee in individuals
with spinal cord injury.
Garland DE,
Adkins RH, Matsuno NN, Stewart CA.
Rancho Los
Amigos Medical Center, Downey, California 90242, USA.
The purpose
of this study was to determine the effects of pulsed electromagnetic
fields on osteoporotic bone at the knee in individuals with chronic
spinal injury. The study consisted of 6 males with complete spinal cord
injury at a minimum of 2 years duration. Bone mineral density (BMD) was
obtained at both knees at initiation, 3 months, 6 months, and 12 months
using dual energy X-ray absorptiometry. In each case, 1 knee was
stimulated using The Bone Growth Stimulator Model 3005 from American
Medical Electronics, Incorporated and the opposite knee served as the
control. Stimulation ceased at 6 months.
At 3 months BMD increased in the
stimulated knees 5.1% and declined in the control knees 6.6% (p <
.05 and p < .02, respectively). By 6 months the BMD returned to near
baseline values and at 12 months both knees had lost bone at a similar
rate to 2.4% below baseline for the stimulated knee and 3.6% below
baseline for the control. There were larger effects closer to the site
of stimulation. While the stimulation appeared useful in retarding
osteoporosis, the unexpected exaggerated decline in the control knees
and reversal at 6 months suggests underlying mechanisms are more complex
than originally anticipated. The authors believe a local as well as a
systemic response was created.
J Spinal
Cord Med. 1999 Winter;22(4):239-45.
***************************************************************************************************************************
PARKINSON'S DISEASE
A drug naive
parkinsonian patient successfully treated with weak electromagnetic
fields.
Sandyk R.
NeuroCommunication Research Laboratories, Danbury, CT 06811, USA.
Brief cerebral application of
picotesla (pT) electromagnetic fields (EMF) has been demonstrated an
efficacious, revolutionary treatment modality for the therapy of
Parkinson's disease (PD) with clinical benefits being evident in all
motor aspects of the disease as well as in nonmotor symptoms such as
mood, sleep, pain, sexual dysfunction, autonomic regulation and
cognitive functions. Since treatment with pT EMF has involved PD
patients who were treated with dopaminergic agents at the time they
received EMF there may have been a synergistic interaction between
dopaminergic drugs and EMF. The present communication concerns a
49-year-old male Parkinsonian patient with stage 3 disability on the
Hoehn and Yahr scale (1967) who, in response to brief extracranial
applications of pT EMF, demonstrated a marked improvement in motor,
depressive symptomatology and cognitive functions and was classified as
stage 1 several weeks later.
This case is remarkable in that the patient did not receive
treatment with dopaminergic drugs prior to or during the course of EMF
therapy. It suggests that (a) pT range EMF may be efficacious as a
monotherapy for PD and should be
considered also as a treatment modality for de novo diagnosed
patients, and (b) application of these EMF improves Parkinsonism by a
mechanism which involves, among others, augmentation of dopaminergic and
serotonergic neurotransmission.
Int J
Neurosci. 1994 Nov;79(1-2):99-110.
***************************************************************************************************************************
Usage Warnings: Persons fitted with pacemakers, defibrillators,
implanted insulin pumps or other electro-medical devices should keep
magnetic therapy products at least 18"(45cm) from the device. Do not
place magnetic therapy products in direct contact with computer discs,
audio/video tapes and credit cards. Pregnant women should consult their
health care professional before using magnetic therapy products. The
information on this Web site is designed for educational purposes only.
It is not intended to be a substitute for informed medical advice or
care. You should not use this information to diagnose or treat any
health problems or illnesses without consulting your pediatrician or
family doctor. Please consult a doctor with any questions or concerns
you might have regarding you or your child's condition. Informational
material and representations have been provided by the manufacturers of
the listed products. In accordance with FDA regulation we do not make
any therapeutic claims regarding the health benefits of any magnetic
products.
[ Bracelets-Women's ]
[ Bracelets - Men's ] [
Necklaces ] [
Pet Collars ] [ Wraps ]
[ Clasps ]
[
Stainless & Titanium ]
|